In simulation, the SWMS on ZnS are intended to investigate the broadband antireflection effectiveness in the spectral array of 1-13 μm by utilizing FDTD technique. The completely matched layer boundary issue is utilized inside the course from the beam propagation (z path). Still, the periodic boundary disorders are set in other two vertical route of beam propagation (x and y way). Moreover, the mesh dimension in simulation grid is picked as fifty nm to attain substantial resolution benefits. The sketch of the simulation SWMS product by using a plane wave propagating with the again of the ZnS is exhibited in Fig.
MDPI and/or maybe the editor(s) disclaim accountability for any injuries to persons or home resulting from any Tips, techniques, Recommendations or items referred to within the information.
Broadband and extensive-angle antireflective subwavelength microstructures on zinc sulfide fabricated by femtosecond laser parallel multi-beam
This Halloween you can thank ZnS for allowing you scare men and women if you have on your ghoulish, glowing deal with paint.
The resulting hydrogen sulfide from this method, in addition to because it happens in normal gas, is converted into elemental sulfur from the Claus course of action. This process entails oxidation of some hydrogen sulfide to sulfur dioxide and after that the comproportionation of the two:[eighty two][83]
Zinc sulfide is usually produced by reacting zinc oxide with hydrogen sulfide, which provides zinc sulfide and water. Another technique for producing the chemical is by heating a solution of zinc sulfate with sodium thiosulfate.
Fig. four). Remarkably, the interface between ZnS and Al:ZnO offers Improved benefits when it comes to wave vector and transition energies, as revealed in Fig. 4. The ZnO/ZnS heterojunction is as a result quite promising, and may be optimized also in see of SPP apps from the IR selection. In Fig. 5, we present actually the SPP wavelength for combined dielectric/metallic interfaces depending on ZnS and ZnO supplies, that we Look at with homogeneous Al:ZnS/ZnS and Al:ZnO/ZnO32 interfaces (all techniques have been listed here thought of of their most stable crystalline composition, i.e. ZB for ZnS and WZ for ZnO). The SPP wavelength defines the duration of a SPP oscillation: our final results demonstrate that distinct conductor/dielectric configurations correspond to distinctive SPP periodicity, specifically Al:ZnS induces lessen (Practically just one fifty percent) SPP wavelengths as opposed to corresponding Al:ZnO-primarily based interfaces; On top of that the larger slope from the curves for ZnO with respect to ZnS for a dielectric points to a larger transported energy for the propagating SPP wave. A proper decision of the material thus permits a tuning of your SPP Qualities with regard to the two activation and intrinsic Vitality with the SPP mode which determine the dimensions of the final optical equipment (e.g. Bragg scatterers).
In summary, the SWMS within the area of ZnS for antireflection in infrared band have already been made by optimizing their geometry to enhance the transmittance in FDTD technique. To begin with, the Original antireflective period of SWMS from the wavelength of eight μm-12 μm is deduced as 3 μm from EMT calculation. Then, the simulated transmittance of SWMS in significantly-infrared band is outwardly rising with enlarging of grating top. The phenomena could be spelled out by linear sleek refractive index gradient formation over the surface of ZnS with greater grating height, resulting to floor Fresnel reflection being drastically suppressed. Finally, the ZnS antireflection band is tunable by managing the period of SWMS, which may be interpreted by WR equation in light industry coupling effect. To confirm the validity of FDTD simulation, the SWMS on ZnS with optimum geometry have been fabricated by making use of femtosecond laser parallel multi-beam.
Zinc sulfide can be employed to supply hydrogen sulfide inside a lab via the response with a solid acid like hydrochloric acid. The reaction proceeds as a result:
Organosulfur compounds are answerable for a lot of the disagreeable odors of decaying organic and natural make any difference. They can be extensively referred to as the odorant in domestic natural fuel, garlic odor, and skunk spray, as well as a ingredient of bad breath odor. Not all organic sulfur compounds odor uncomfortable in any way concentrations: the sulfur-containing monoterpenoid grapefruit mercaptan in modest concentrations is the characteristic scent of grapefruit, but contains a generic thiol odor at larger sized concentrations. Sulfur mustard, a powerful vesicant, was used in Globe War I for a disabling agent.[55]
g. ZnO20, SiC21 and BeO22, is connected to the minimized steadiness of the hexagonal vs zincblende structures, and to The reality that sizeable differences concerning the two lattices seem only at third neighbors. However, the presence of a spontaneous polarization may well induce related results in the main levels of strongly non-adiabatic growth processes, including favoring just one lattice about the other, specifically for thin films and nanostructures6.
^ But impure samples have an odor much like that of matches. A robust odor identified as "scent of sulfur" in fact is provided off by a number of sulfur compounds, which include hydrogen sulfide and organosulfur compounds. ^ A determine of sulfur's melting issue in a hundred and fifteen.21°C were based on two laboratories with the US Section of Vitality (Jefferson Lab and Los Alamos Nationwide Lab).[12] Greenwood and Earnshaw state that at fast heating for microcrystalline α-S8 the melting issue in one hundred fifteen.
Zinc was distinctly recognized like a steel underneath the designation of Yasada or Jasada during the healthcare Lexicon ascribed on the Hindu king Madanapala (of Taka dynasty) and published about the calendar year 1374.
All pure sphalerite consists of concentrations of various impurities, which frequently substitute for zinc in the cation situation in the read more lattice; the most typical cation impurities are cadmium, mercury and manganese, but gallium, germanium and indium could also be present in reasonably higher concentrations (hundreds to Many ppm).
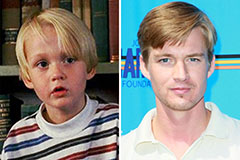 Mason Gamble Then & Now!
Mason Gamble Then & Now! Patrick Renna Then & Now!
Patrick Renna Then & Now! Romeo Miller Then & Now!
Romeo Miller Then & Now! Lisa Whelchel Then & Now!
Lisa Whelchel Then & Now! Nicki Minaj Then & Now!
Nicki Minaj Then & Now!